Here’s What the OIG Added to its Work Plan in December 2023
Happy Compliance New Year!
But before we get too far into 2024, let’s take a quick look back at 2023 because the U.S. Department of Health and Human Services Office of Inspector General (OIG) added some new items to their Work Plan in December.
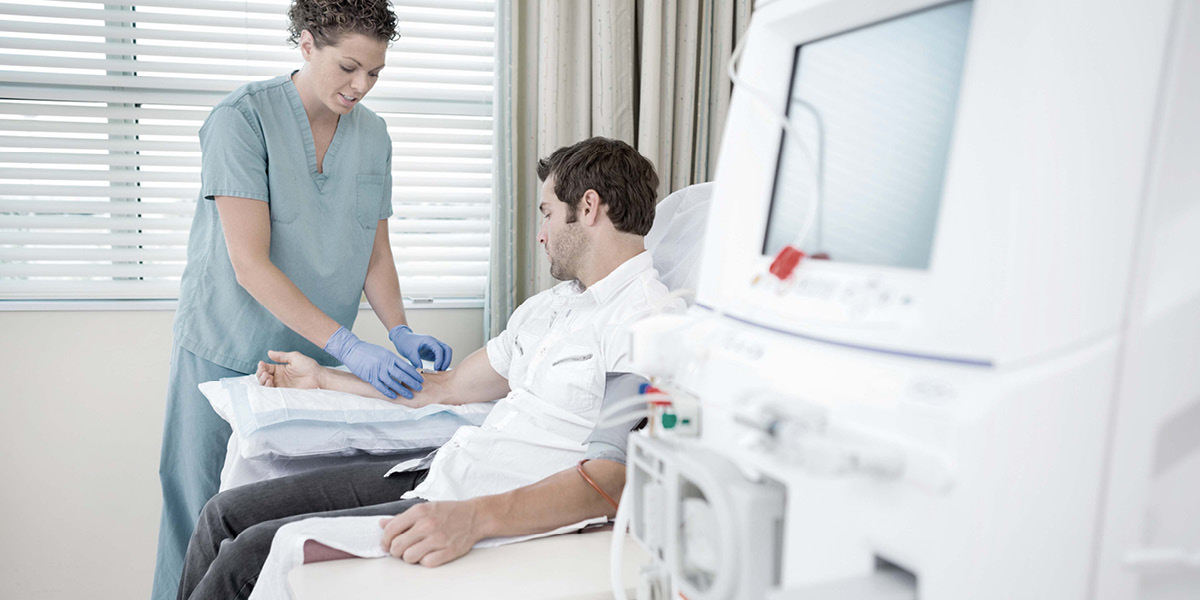
Medicare Home Dialysis
Taking care of Medicare patients with End-Stage Renal Disease (ESRD) is about as old as the Medicare program itself, since it has been an important way that individuals could qualify for Medicare. And a lot of Medicare funding is spent on ESRD services.
Medicare Part B covers outpatient dialysis services for beneficiaries with ESRD. Such outpatient treatments can be provided in an outpatient or home setting and must be monitored by certified ESRD facilities.
Over the years, OIG has completed quite a bit of work assessing compliance with dialysis requirements. A few examples of their audits can be found here and here.
Some of the issues they have previously identified include:
-
- Comprehensive assessments or plans of care did not meet Medicare requirements
- Dialysis treatments were not completed
- Dialysis services were not documented
- Beneficiaries’ height or weight measurements did not comply with Medicare requirements
- The medical record did not have a monthly progress note by a physician or other qualified professional
- No documentation of the dispensing or administration of medication billed
- Medication billed exceeding physician-prescribed amounts
For this work plan item, the OIG plans to review claims for Medicare Part B home dialysis services provided to ESRD patients to determine whether such services complied with Medicare requirements.
They will also review the impact of home dialysis services on enrollees and whether enrollees' quality of care could be affected.
Audit of Tribal Controls Over Construction Program Costs
Through legislative action, the U.S. Congress has allocated $3.5 billion to the Indian Health Service (IHS) Sanitation Facilities Construction (SFC) Program to provide American Indian and Alaska Native homes and communities with essential water supply, sewage disposal, and solid waste disposal facilities.
Frequently, when there is significant tax-payer funding involved (such as in this case), the OIG will perform audits to ensure there are sufficient internal controls associated with the funding and projects.
So, it should come as no surprise that the OIG is announcing they will do just that. They will examine the controls in place over SFC program construction projects that are managed by an agreement between IHS and a Tribe, or managed by a Tribe that has assumed sole responsibility for a project.
They’ve identified their objectives for the audits will focus on the controls that were implemented for awarding, monitoring, and reporting SFC program projects according to applicable Federal and Tribal requirements.
Medicare Payments for Emergency Services
Hospital emergency departments are defined as an organized hospital-based facility for the provision of unscheduled or episodic services to patients who present for immediate medical attention.
The Current Procedural Terminology (CPT) and Healthcare Common Procedure Coding Systems (HCPCS) have unique codes that should only be used when Medicare beneficiaries are seen in an emergency department as described by the codes' definitions. When providers submit claims for services, they are required to identify the site of service through what are known as Place of Service (POS) codes. The POS can influence the amount Medicare reimburses providers.
With this audit, the OIG plans to determine whether Medicare appropriately paid hospitals and physicians for emergency department services provided in nonemergency department sites of service.
It should be noted, we reported previously that the OIG was going to perform and audit on the medical necessity and appropriateness of the evaluation and management codes physician have reported for emergency department services. That audit is expected to be released sometime in 2024 (see: https://oig.hhs.gov/reports-and-publications/workplan/summary/wp-summary-0000612.asp)
Clinical Trial Results Reported to ClinicalTrials.gov.
Clinical trials are essential for advances in medicine and healthcare. Such trials help determine whether interventions are safe and effective. With this in mind, it is important to have transparency with the public about clinical trial results. The website clinicaltrials.gov is a place where some information about past, current and future clinical trials is shared.
The Food and Drug Administration (FDA) is responsible for ensuring that results of applicable clinical trials are reported to ClinicalTrials.gov within one year of the completion date.
FDA monitors compliance with reporting requirements through various methods, including evidence collected during inspections conducted as part of FDA's Bioresearch Monitoring Program. The FDA may notify a responsible party that has not met reporting requirements and allow 30 days to remedy the noncompliance. If a responsible party remains noncompliant, the FDA may initiate a corrective action, including seeking civil monetary penalties.
The OIG’s audit in this area will be designed to determine whether the FDA notified responsible parties of noncompliance with the requirement to submit the results of applicable clinical trials in accordance with Federal requirements.
Pharmacy Records
Pharmacies have also not been left out of the compliance fun.
Medicare Part D plays an important role in drug benefits for Medicare beneficiaries. Medicare Part D plan sponsors are required to submit Prescription Drug Event (PDE) records to the Secretary of Health and Human Services in order to determine payments to the plans. PDE records are summary records of pharmacy drug claims.
This Work Plan item announces the OIG’s intent to audit select pharmacies so they can determine whether PDE records were adequately supported by inventory purchases and complied with applicable Federal requirements.
Conclusion
Healthicity hopes you had a wonderful holiday season and that you will have a successful New Year. Just in case you missed these during the holiday hustle and bustle (or other December additions to the OIG’s work plan), make sure to get back into the swing of things and review these recent additions.
To download this blog post as a pdf, click the button below.


Questions or Comments?